Elements compounds and mixtures quiz – Embark on an enlightening journey with our Elements, Compounds, and Mixtures Quiz. Delve into the fascinating world of chemistry as we unravel the fundamental concepts, properties, and applications of these substances, providing a thorough understanding of their significance in our daily lives and various industries.
Through engaging discussions, illustrative examples, and thought-provoking questions, this quiz promises to enhance your knowledge and deepen your appreciation for the intricate tapestry of chemical elements, compounds, and mixtures.
1. Definitions and Concepts
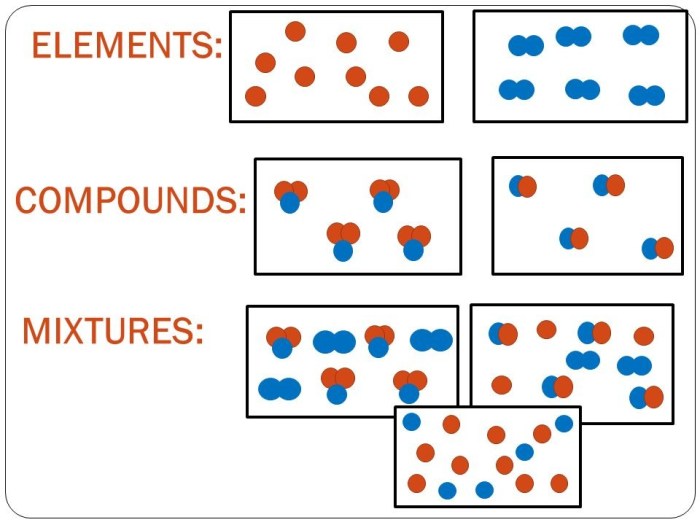
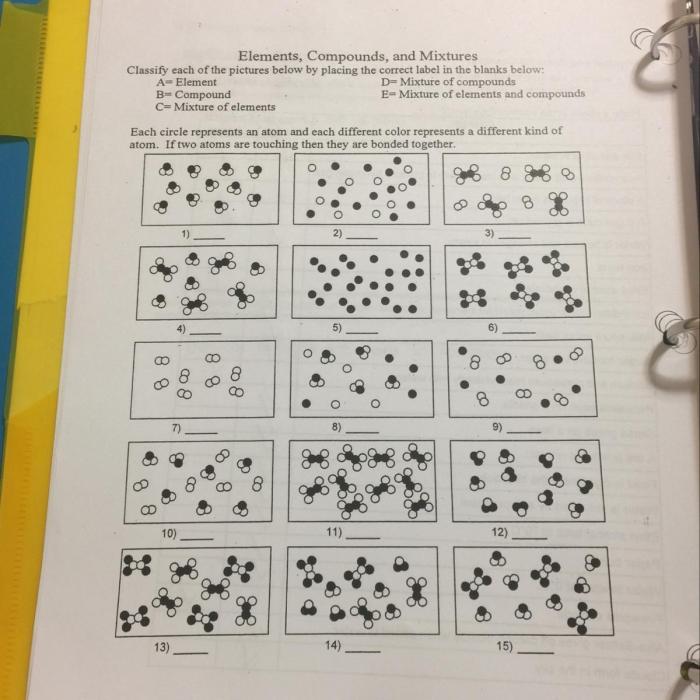
In chemistry, matter can be classified into three fundamental categories: elements, compounds, and mixtures. These substances differ in their composition and properties, and understanding their distinctions is crucial for comprehending the field.
1.1 Elements
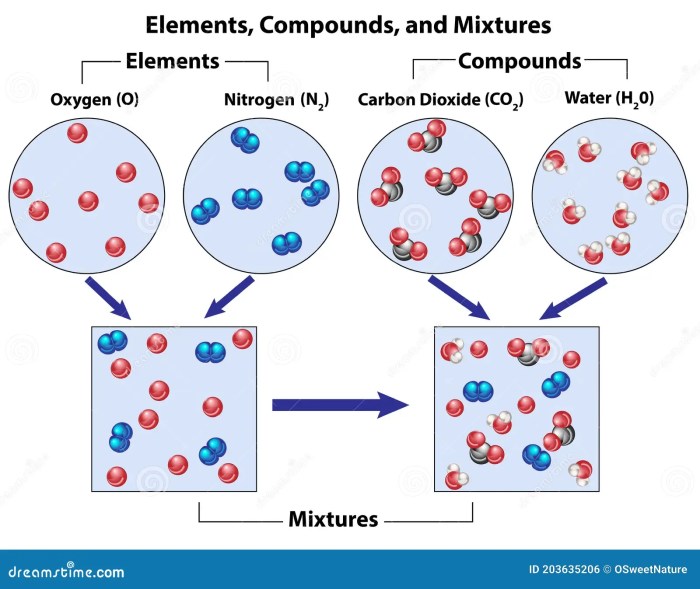
Elements are the simplest form of matter and cannot be broken down into simpler substances through chemical means. They are composed of only one type of atom, which is characterized by its unique atomic number and electron configuration. Examples of elements include hydrogen (H), oxygen (O), and gold (Au).
1.2 Compounds, Elements compounds and mixtures quiz
Compounds are formed when two or more elements combine chemically in fixed proportions. They consist of molecules, which are groups of atoms held together by chemical bonds. Compounds have unique properties that differ from those of their constituent elements. Examples of compounds include water (H2O), carbon dioxide (CO2), and sodium chloride (NaCl).
1.3 Mixtures
Mixtures are combinations of two or more elements or compounds that are not chemically bonded. The components of a mixture retain their individual properties and can be separated by physical means, such as filtration or distillation. Examples of mixtures include salt water (a mixture of water and salt) and air (a mixture of nitrogen, oxygen, and other gases).
Q&A: Elements Compounds And Mixtures Quiz
What is the primary distinction between elements, compounds, and mixtures?
Elements are pure substances composed of a single type of atom, while compounds are substances formed by the chemical combination of two or more elements in fixed proportions. Mixtures, on the other hand, are combinations of two or more substances that retain their individual properties and can be physically separated.
How are elements formed?
Elements are naturally occurring substances that cannot be broken down into simpler substances through chemical means. They are the fundamental building blocks of all matter and are arranged in the periodic table based on their atomic number.
What are some common examples of compounds?
Water (H2O), table salt (NaCl), and carbon dioxide (CO2) are all examples of compounds. Compounds exhibit unique properties that differ from their constituent elements due to the chemical bonds formed between them.
How can mixtures be separated?
Mixtures can be separated based on the physical properties of their components. Common methods include filtration, distillation, and chromatography, which utilize differences in size, boiling point, or chemical affinity to isolate individual substances.